21 CFR Part 11 establishes the standards by which the US Food and Drug Administration will accept electronic records and electronic signatures as the equivalent of paper records and handwritten signatures. Compliance requires positive identification of the person creating or modifying data records, as well as the use of audit trails for the data and system parameters affecting collection and management. Applications in the lab must provide adequate security, protection, and audit trails for collecting and managing data.
LabSpeed Offers a Complete 21-CFR Part 11 Solution
It is extremely resource intensive to add 21 CFR to an Instrument application. To ease the burden, Topos offers a set of 21-CFR software libraries, which the instrument manufacturer can reference in its own software. Utilizing these libraries, a programmer can quickly implement the signing of sample data, log 21 CFR events, and verify user passwords.
Utilizing the same libraries, LabSpeed OEM does the rest to ensure compliance. LabSpeed queries, validates and displays 21 CFR data records, adds signatures for review and approval, and displays the Audit log.
The diagram below indicates the roles the Instrument software and LabSpeed play in the overall 21-CFR Part 11 solution:
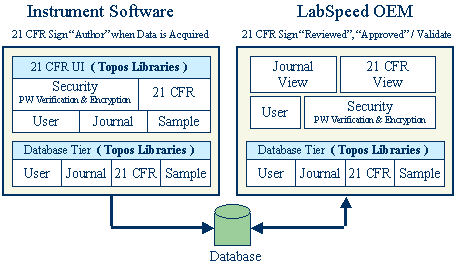
With the LabSpeed libraries, the instrument manufacturer can deliver a ready-made 21-CFR Part 11 authoring solution in a fraction of the time it would take if developed in-house.